A new treatment for postoperative pain management.
Paracetamol 1000 mg and ibuprofen 300 mg in 100 mL solution for infusion.
Combogesic® IV’s patented 3.3 to 1 paracetamol to ibuprofen ratio has been proven to provide significantly better pain relief than the same dose of either paracetamol IV or ibuprofen IV alone.1
Combogesic® IV was developed to provide a combination analgesic therapy with the administration of a single formulation in postoperative patients unable to receive oral analgesics.
Effective treatment for postoperative pain management.
- New clinical data demonstrates that Combogesic® IV provides significantly more analgesia than comparable doses of paracetamol IV, ibuprofen IV and placebo.1
- All study medicines were administered to patients intravenously over 15 minutes every 6 hours over a 48-hour period, for a total of 8 doses.
- Combogesic® IV provided significantly superior pain relief than comparable doses of ibuprofen or paracetamol, and placebo.1
Double the pain relief1
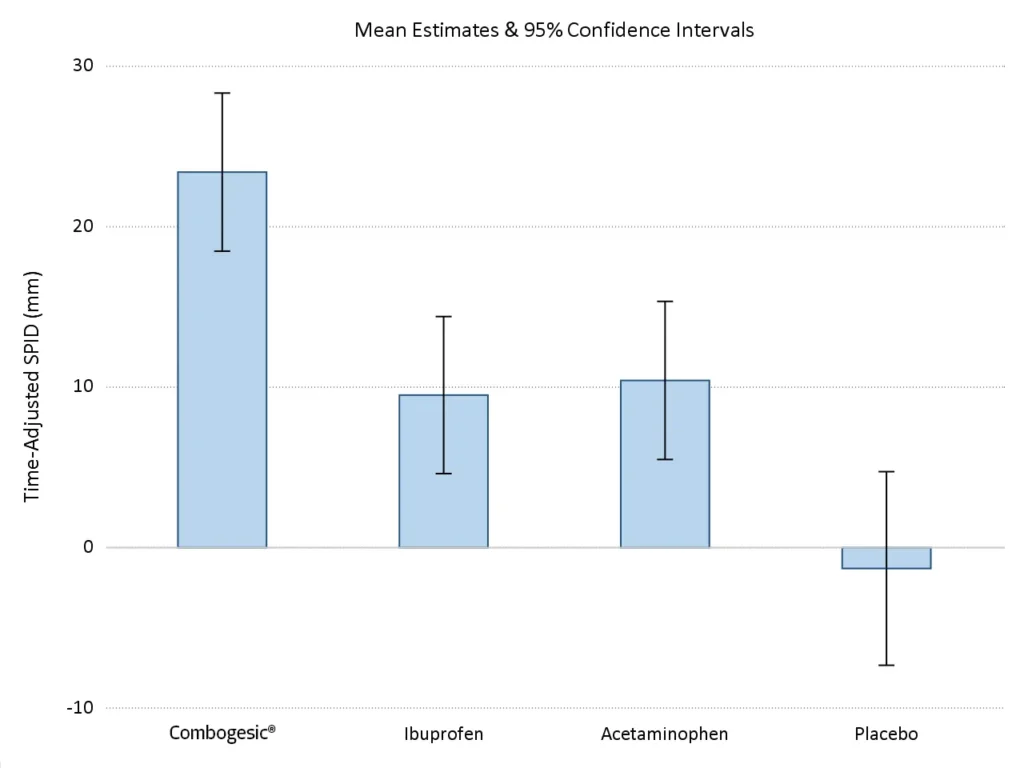
Fig 1: Time-adjusted SPID48 up to first dose of rescue medication
The amount of pain relief provided by Combogesic® IV was approximately DOUBLE that of paracetamol IV and ibuprofen IV alone*,1
Combogesic® provided the greatest amount of pain relief, far exceeding the amount required for a clinically significant difference, with all pairwise comparisons being highly significant.*
*Based on time-adjusted SPID48, calculated from VAS pain intensity scores recorded up until the time of consumption of the first dose of rescue.
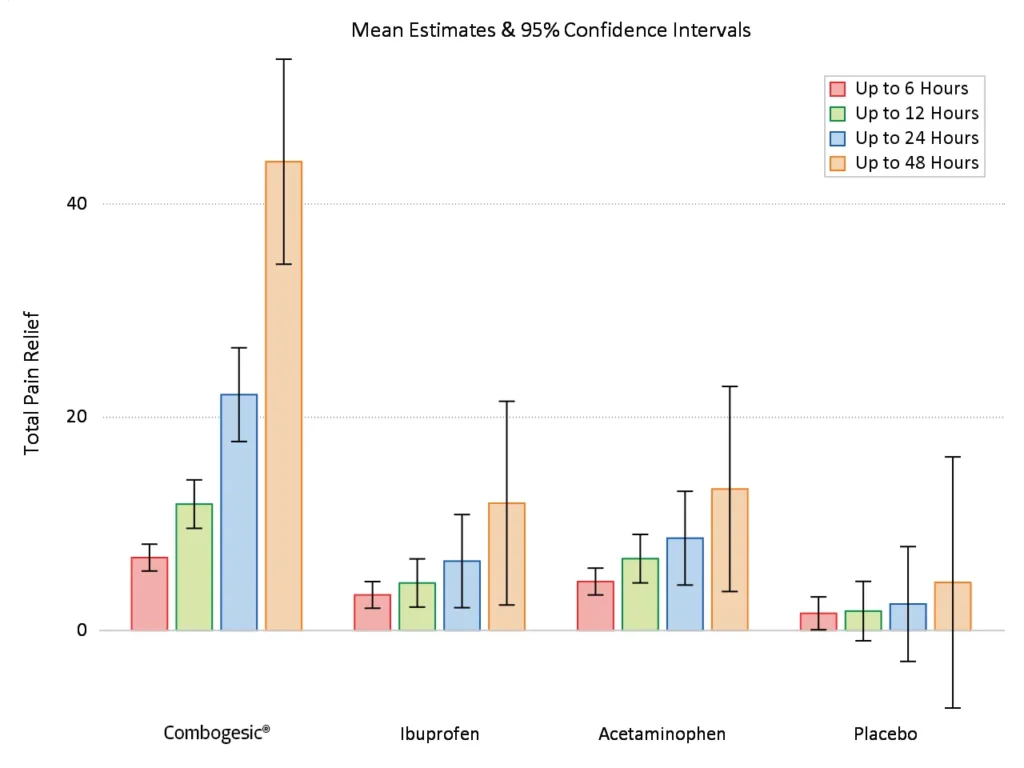
Greater than all three comparators1
Combogesic® IV provided greater pain relief over the first 6, 12, 24 and 48 hours treatment period than all three comparators**,1
Combogesic® IV is an effective, opioid-free, pain management tool, with significantly greater pain relief than either of its mono components over the first 6, 12, 24 and 48 hours.**
**As measured by Total Pain Relief (TOTPAR) calculated from the area under the curve of pain relief scores over a specified time interval calculated by the linear trapezoidal rule, up until the first pre-rescue pain relief. There is no time adjustment for TOTPAR.
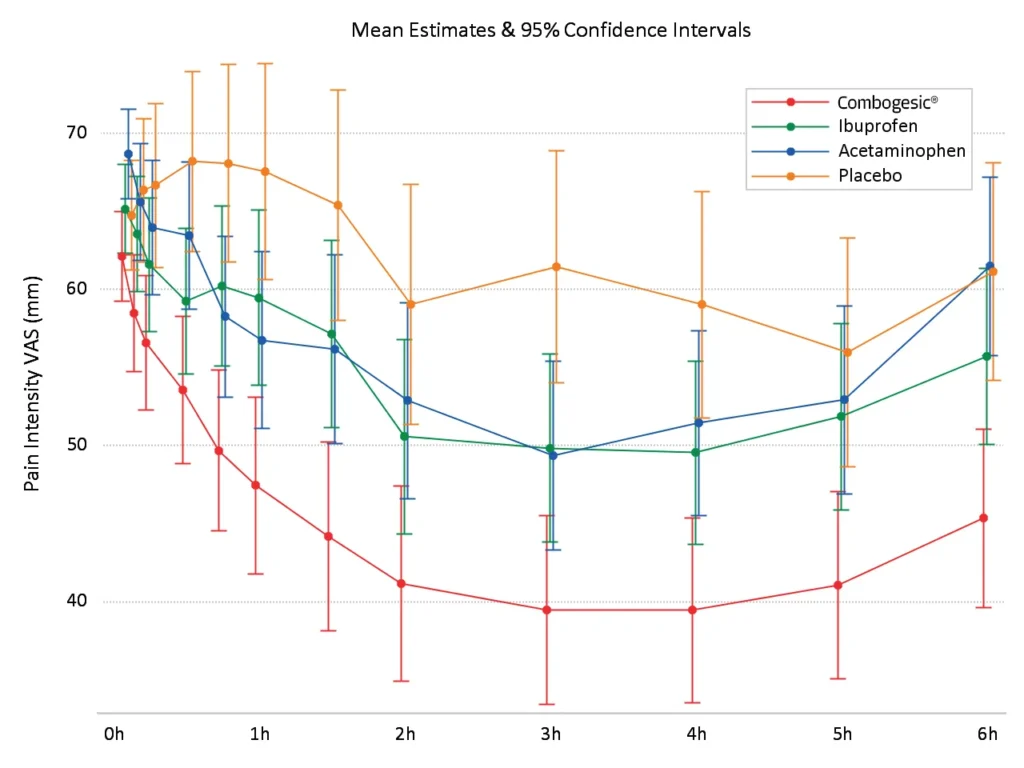
Providing sustained pain relief over the first dose
Combogesic® IV provided significantly greaterpain relief than all three treatment groups at the majority of scheduled time points*,1
Providing sustained pain relief over the first dose
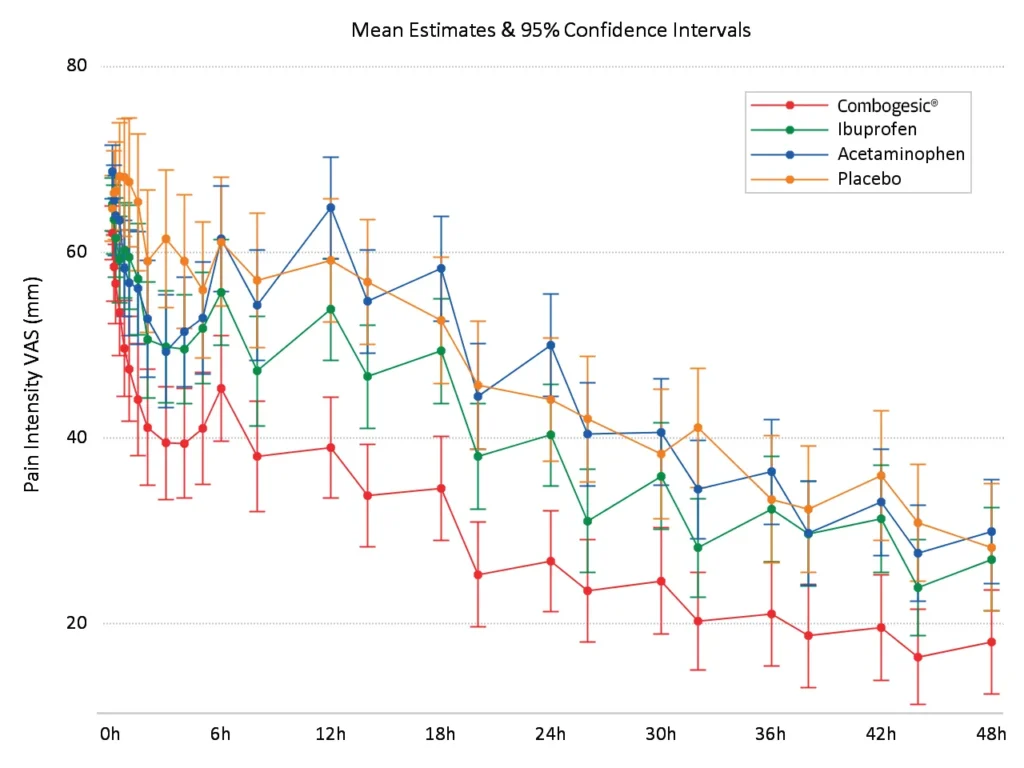
*According to VAS pain intensity, Pain Intensity Differences and Pain Relief scores.
Providing sustained pain relief over 48 hours
Combogesic® IV provided significantly greaterpain relief than all three treatment groups at the majority of scheduled time points*,1
Providing sustained pain relief over 48 hours
No compromise in safety.
- The postoperative safety profile of Combogesic® IV is equivalent with that of paracetamol IV or ibuprofen IV and no novel adverse events were reported.1
- Combogesic® IV can be given after surgery and continued in the postoperative period as a component of multimodal pain management.1
From hospital to home – easy IV to tablet switch.
The Combogesic® IV treatment group was found to have:
- Significantly reduced odds of requiring opioid analgesia for breakthrough pain than all three comparators.1
- A longer time to the first dose of breakthrough analgesia than all three comparators.1
- Significantly lower consumption of oxycodone breakthrough analgesia than all three comparators.1
Why is this important?
- Immediate-release formulations of prescription opioids have been described as a gateway to addiction.2
- The potential harm from extended opioid treatments and escalating usage of opioid based treatments has been a strong focus of pain medicine research and forums.
- Effective pain solutions are needed that allow for an easy opioid-free IV to tablet switch for pain management in the clinic and at home.
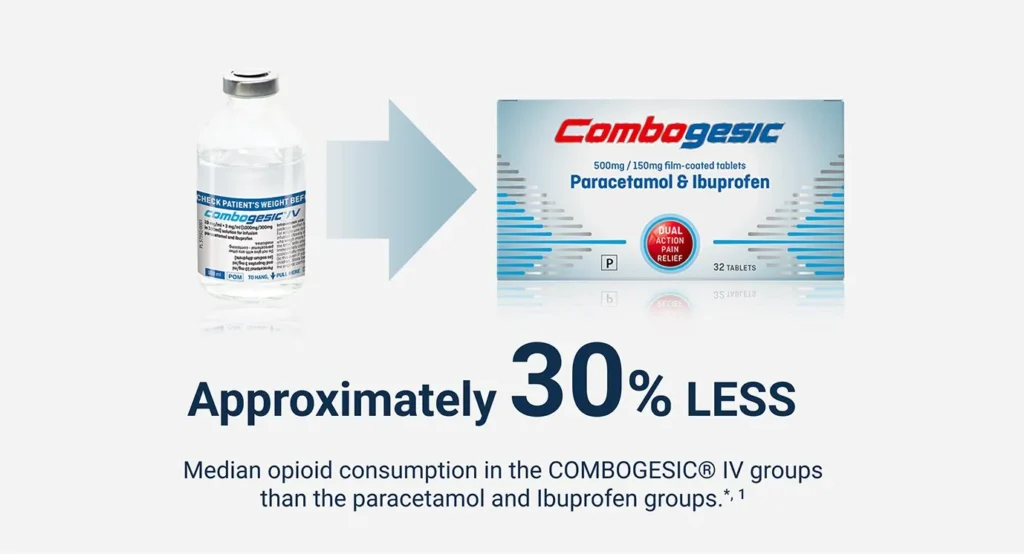
*Based on the total oral Morphine Milligram Equivalent (MME) dose of all rescue medication consumed over the full 48 hour study period.
Resources
Prescribing information
United Kingdom
Download (PDF)
Northern Ireland
Download (PDF)
Combogesic IV Article
Download (PDF)
Patient resources
United Kingdom
Download (PDF)
Northern Ireland
Download (PDF)
References
1. Daniels, S.E, Playne, R., Stanescu, I., Zhang, J., Gottlieb, I.J, Atkinson, H.C. (2019). Efficacy and safety of an intravenous acetaminophen/ibuprofen fixed-dose combination after bunionectomy: A randomized, double-blind, factorial, placebo controlled trial. Clinical Therapeutics 41 (10): 1952-1965. Research sponsored by AFT Pharmaceuticals.
2. FDA Commissioner Scott Gottlieb. September 28, 2017. “FDA Takes Important Steps to Stem the Tide of Opioid Misuse and Abuse | FDA Voice.”. Retrieved https://www.fda.gov/news-events/fda-voices-perspectives-fda-leadership-and-experts/fda-takesimportant-steps-stem-tide-opioid-misuse-and-abuse
Reporting adverse events
Adverse events should be reported. Reporting form and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to AFT Pharma UK Ltd on 01934 838820.
Please be aware that this website contains promotional information about AFT Pharmaceuticals medicines and services. Some of this may not be directly relevant to your scope of practice and it is your own decision whether you choose to view this information.

